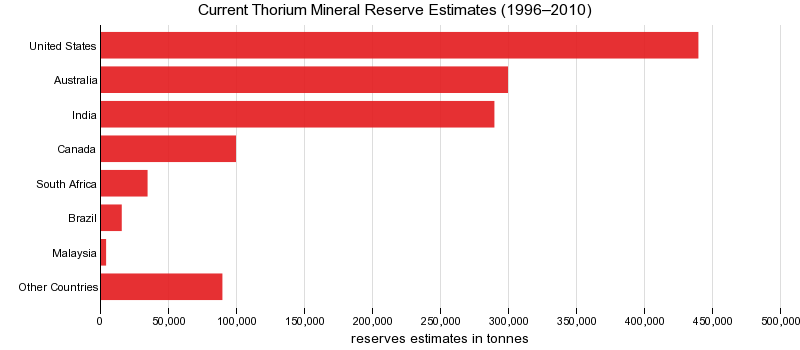
This chart show current Thorium mineral reserve estimates (1996–2010).
Thorium consumption worldwide is relatively small compared with that of most other mineral commodities. There was no domestic production of thorium reported in 2009.
What is Thorium?
Thorium is a naturally occurring radioactive chemical element, found in abundance throughout the world. Thorium atoms (symbol Th) have an atomic number of 90, with 90 protons and 90 electrons, of which 4 are valence electrons. It was discovered in 1828 and named after Thor, the Norse god of thunder. (from Wikipedia)
In nature, thorium is found as thorium-232 (100.00%). Thorium decays slowly by emitting an alpha particle. The half-life of thorium-232 is about 14.05 billion years. It is estimated to be about three to four times more abundant than uranium in the Earth's crust. It is a by-product of the extraction of rare earths from monazite sands. The formerly widespread uses of thorium, for example as a light emitting material in gas mantles or as an alloying material in several metals, have decreased due to concerns about its radioactivity.
Thorium-232 was used for breeding nuclear fuel – uranium-233, for example, in the molten-salt reactor experiment (MSR) conducted in the United States from 1964 to 1969. Most of the initial test reactors were closed down. However, countries including Russia, India, and recently China, have plans to use thorium for their nuclear power, partly because of its safety benefits.
15 years ago