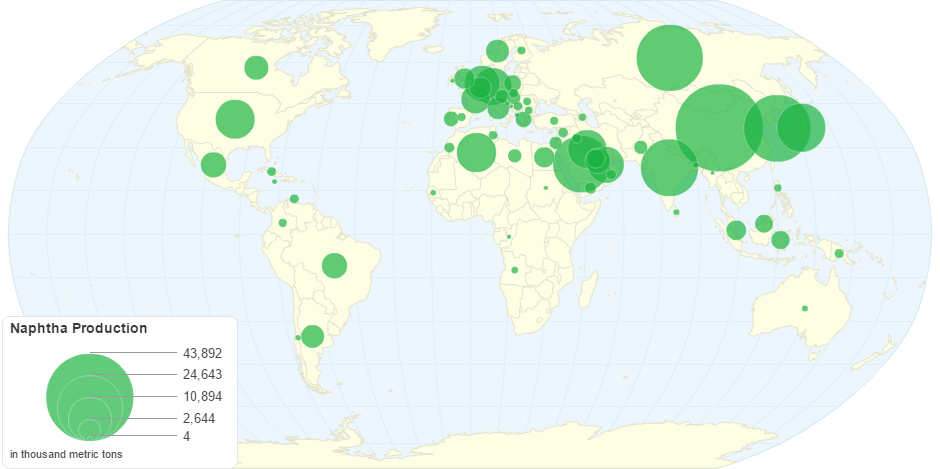
This chart shows Naphtha Production by Country.
Naphtha is a general term that has been used for over two thousand years to refer to flammable liquid hydrocarbon mixtures. Mixtures labelled naphtha have been produced from natural gas condensates, petroleum distillates, and the distillation of coal tar and peat. It is used diversely in different industries and regions to refer to gross products like crude oil or refined products such as kerosene.
Various qualifiers have been added to the term "naphtha" by various sources in an effort to make it more specific:One source differentiates by boiling point:Light naphtha is the fraction boiling between 30 °C and 90 °C and consists of molecules with 5–6 carbon atoms. Heavy naphtha boils between 90 °C and 200 °C and consists of molecules with 6–12 carbons.
Another source differentiates light and heavy based on hydrocarbon structure:Light a mixture consisting mainly of straight-chained and cyclic aliphatic hydrocarbons having from five to nine carbon atoms per molecule. Heavy a mixture consisting mainly of straight-chained and cyclic aliphatic hydrocarbons having from seven to nine carbons per molecule.
The MSDSs for various vendors are also indicative of the non-specific nature of the product and reflect the considerations due for a flammable mixture of hydrocarbons: flammability, carcinogenicity, skin and airway irritation, etc. People can be exposed to naphtha in the workplace by breathing it in, swallowing it, skin contact, and eye contact.
The Occupational Safety and Health Administration has set the permissible exposure limit for naphtha exposure in the workplace as 100 ppm over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit of 100 ppm over an 8-hour workday. At levels of 1000 ppm, 10% of the lower explosive limit, naphtha is immediately dangerous to life and health.
9 years ago